Toluene
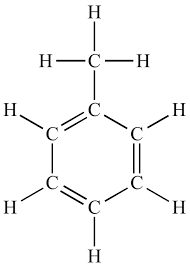
Toluene (C7H6) is a colorless liquid that can be distinguished by a smell resembling that of paint thinners. Also known as methylbenzene, this inexpensive substance is easy to produce and is widely used as a solvent in industrial processes. It is also used as an octane booster in gasoline and can be found in flues, solvents, and resins.
Exposure to moderate levels of toluene causes a variety of side-effects including tiredness, confusion, weakness, memory loss, and nausea. These symptoms are generally not severe and subside when exposure is stopped. However, inhalation of high levels of toluene may lead to a loss of consciousness or death.
EPA has set the maximum contaminant level for toluene in drinking water at 1 mg/L or 1 ppm.
Oxidation Information:
Reaction with Ozone: C6H5CH3 + 18 O3 ---> 7 CO2 + 4 H2O + 18 O2
Number of O3 molecules consumed per molecule of toluene: 9
Molecular weights: Ozone=18 Toluene=92.14
Grams of ozone required to fully oxidize 1 gram of toluene: 4.69
Calculation: 1 gram toluene / 92.14 = 0.0108530497 * 48 * 9 moles of ozone = 4.69 grams of ozone
















