Enhancing Ozone Monitoring Accuracy with Eco Sensors SM-7 Sensor
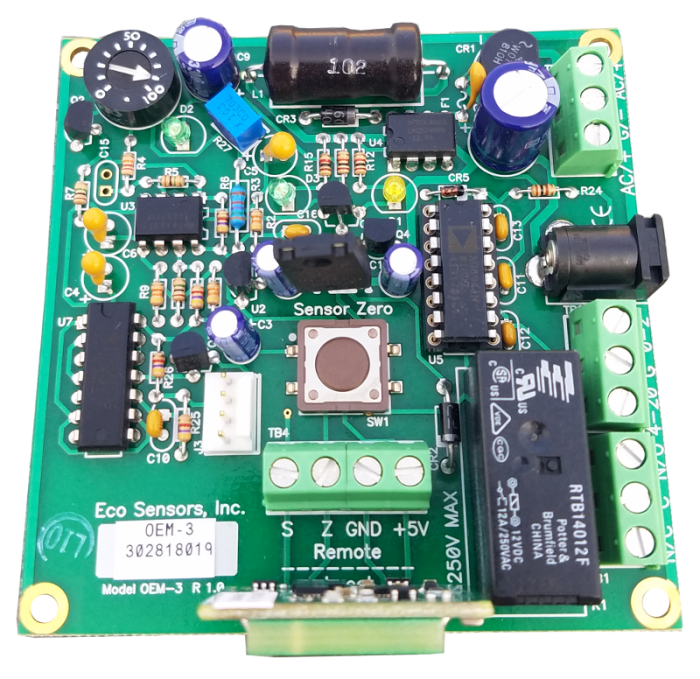
In industrial and commercial settings where ozone levels need precise monitoring, Eco Sensors’ SM-7 Sensor emerges as a versatile and accurate solution. Designed to meet …
Enhancing Ozone Monitoring Accuracy with Eco Sensors SM-7 Sensor Read More »