Ozone is commonly dissolved into water for water treatment, or for water to carry ozone for other jobs like sanitizing surfaces or produce. In fact, many of the applications of ozone require ozone to be dissolved into water.
Ozone is normally produced as a gas from oxygen in air, or concentrated oxygen. That gas must then be dissolved into water. As ozone is partially soluble into water it is possible to dissolve ozone into water but does require the proper equipment to be efficient.
As producing ozone requires equipment and electricity, there is a cost for every gram of ozone produced. Therefore, there is value tin ensuring every gram possible is dissolved efficiently into water with as few losses as possible.
How easily ozone dissolves into water is based on the solubility of ozone into water. Learn more about ozone solubility here.
The solubility of ozone into water is the ratio of ozone that will dissolve into water, and the overall capacity to dissolve ozone into water. The solubility of ozone into water is based on Water Temperature, Ozone Concentration, and Water Pressure. See image below for a visual example of how ozone concentration and temperature affect ozone solubility.
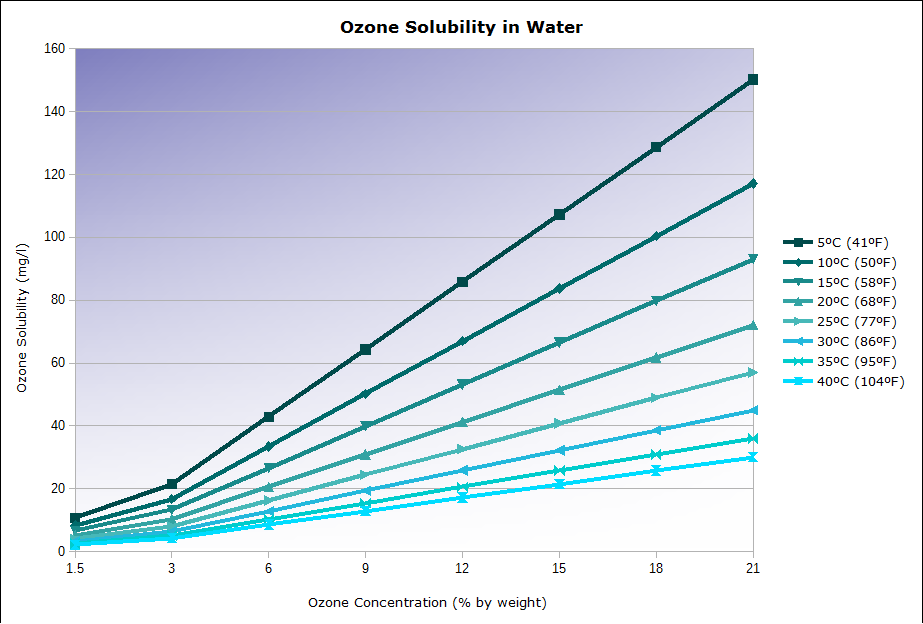
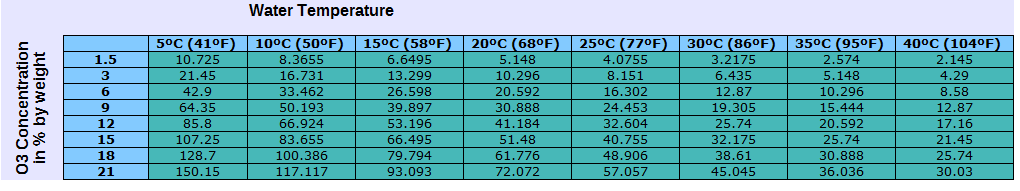
The chart above shows the maximum ozone concentration in water possible for a given water temperature and ozone concentration. The higher the max ozone concentration, the easier it will be to dissolve ozone into water. The lower the max concentration (solubility) the harder it will be to dissolve ozone into water as the excess ozone will simply off-gas and be wasted.
See this information in chart form:
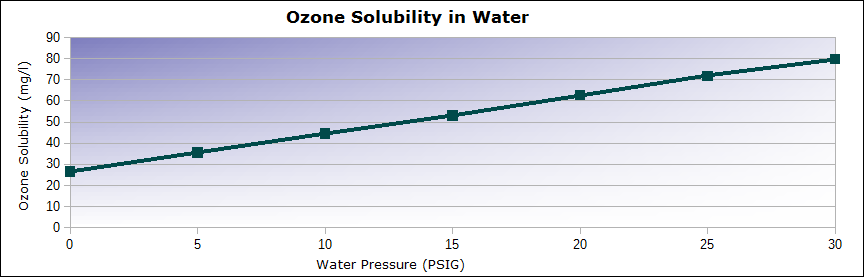
Pressure also affects ozone solubility. Increased water pressure will push ozone gas into the water more efficiently. This is a linear affect, more pressure = higher solubility factors. See chart below for visual example.
Summary:
- Higher ozone concentrations = higher dissolved ozone levels in water
- ozone generators that produce ozone at higher concentrations will dissolve ozone into water more efficiently than those that produce the same amount of ozone at a lower concentrations
- ozone production in g/hr is not as important as concentration
- Look for higher % by weight, or g/m3 numbers
- Lower water temperatures = higher dissolved ozone levels in water
- Colder water dissolves ozone easier
- adding ice cubes to small volumes of water will increase dissolved ozone levels
- Higher water pressure = higher dissolved ozone levels in water
- If bubbling ozone into water use taller columns, the water at the bottom of the column (where the diffuser is) will be under higher pressures
- when using venturi systems ensure the contact tank is under some pressure.
Learn more about ozone solubility and how you can apply this information to your application at the link below:

I discovered your blog site on google and check a few of your early posts. Continue to keep up the very good operate. I just additional up your RSS feed to my MSN News Reader. Seeking forward to reading more from you later on!…
Pure water will dissolve ozone better than water that is saturated with other chemicals such as hardness. This may require using water at the end of a wastewater treatment facility to be ozonated then added where needed, especially if it can be cooled first.
Similar with industrial cooling towers where cooled makeup water is ozonated then added to the cooling water.
Any evidence to support your ridiculous claims?
I’m interested in an ozone infusion system for use in an off grid application to produce potable water from a spring or river. Preferably i want to treat water before it is pumped into a storage tank but it could be acceptable to dose in a storage tank with appropriate controls.
What are best methods for verifying water quality such as conductivity, pH and what temperatures work best?
The best test for water initially is to send a sample of your water to a water testing lab and have a standard “drinking water test” performed on that water. This will tell you what is in the water. From there, when using ozone for water treatment we are most concerned about ORP. A low-cost ORP monitor could be obtained to measure ORP and ensure the ozone system is performing the proper job.
I have one problem regarding ozone treatment.
RO treated water feed into a tank (10 m3) and ozone is circulating in that tank. Initially ozone concentration reach target level (800 mv). Then stop ozone circulation at night . Next morning start again ozone circulation but ozone concentration does not reach target level for long time circulation. If we fill up new RO water into that tank ozone goes up again.
Could you advice why it is happening ?. .
Lwin,
This is a phenomenon we have seen before in Ulta-Pure water applications. There are a variety of reasons this may happen, and the fundamentals are complicated. Glad to discuss this if you with to contact our office directly.
In summary, you are likely conductivity in water going up, correlating with a lower dissolved ozone level in water with all other parameters remaining consistent. This is likely due to higher levels of CO2 of NO2 (and further reactions of each) in the water. Ozone is reacting with the resulting compounds and being consumed. I’m sure we can help, we would just need a bit more info on your set-up to determine the root cause, let us know if you have further questions.
WE HAVE USED 5 GMS/HOUR OZONE SYSTEM FOR A FLOW OF 1500 LITRES/HOUR WASTE WATER. PRESSUE DROP ACROSS THE EDUCTOR NOZZLE IS 1.5 BAR. I AM NOT HAPPY WITH THE SOLUBILITY LEVEL. WHAT IS WRONG ?
Can you please clarify?
Are you not happy with the solubility level (excess amounts of ozone are off-gassing from the water, not getting dissolved)?
Or, are you not happy with the final dissolved ozone levels (all the ozone is consumed by the water leaving no residual in the water)?
We would also want details on the 5 g/hr ozone generator. What is the gas flow-rate and ozone concentration from this machine?