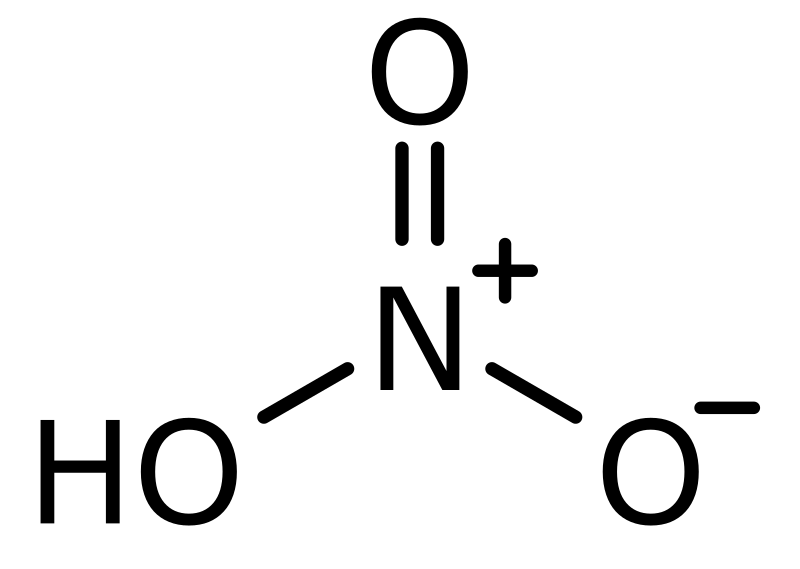
Ozone production requires oxygen in the feed gas. Air is 21% oxygen, so when air is used as a feed gas for an ozone generator, some of that oxygen O2 is used to make ozone O3. Air also has lots of nitrogen N and water H2O – the ingredients of nitric acid HNO3. The presence of ozone with water and nitrogen readily makes nitric acid. An ozone generator fed with ambient air will make nitric acid. When the air is warm and the humidity is high, an ozone generator will make lots of nitric acid.
In the ambient air type ozone generators often used for odor remediation or blowing some ozone into a room, provision is made to remove plates and screens from the generator for periodic cleaning. (picture of this type of generator) Using these generators in an air conditioned space or with a dehumidifier greatly extends the time between cleaning the nitric acid and dust buildup from the ozone generating components.
The type of generators that force air through a tube with a little air pump for bubbling in water are not so easily cleaned and the acid starts to accumulate or run out of the ozone port. (picture of OZX-300) Since the nitric acid is something we don’t want from an ozone generator, you can simply dispose of the acid when it collects in the tubing (disposal method), but the preferred way is to prevent the acid production by removing the water from the feed gas (prevention method).
Nitric Acid Disposal Method
With a small, single cell ozone generator making a couple hundred mg/hour of ozone, a vertical orientation with the ozone tube straight down to the point of injection into water is a way to address the nitric acid problem by disposing of it. Having a filter on the air inlet will help prevent dust particles from entering, building up on droplets of nitric acid, and clogging the generator. This solution may extend the life of the ozone generator, but ozone is being used up to make nitric acid which reduces ozone production. Also, it is inevitable that droplets of liquid will collect dust particles, accelerate corrosion, and eventually result in failure of the system. The nitric acid along with contaminants in the air eventually make a conductive path for the high voltage electrode to a metal fitting or the water. This can result in an electrical shock hazard and eventual burnout of the high voltage driver circuit.
The disposal method of dealing with nitric acid can work for awhile in the realm of small, single cell ozone generators that feed ozone directly to water. It is very simple, and when it fails, the generator and tubing is simply replaced. But this method is not acceptable to anyone in the ozone industry.
Nitric Acid Prevention Method
In the ozone industry, rule #1 is to prevent as much as possible the entry of moisture into the ozone generating cell. The ambient air we are used to living in has a surprising amount of water in it. If your generator is using 1 LPM of ambient air, then a total of 43,200 liters of air passes through it after a month of continuous operation. If this air is 100 deg F at 100% humidity, nearly a half a gallon of water will have passed through the ozone generator. It is not uncommon to spend as much on the feed gas preparation as it is on the ozone generator itself to ensure many years of reliable ozone production.
The following are two simple, low cost options that will greatly extend the life of any air fed ozone generator:
1) Use a length of tubing on the inlet of your ozone generator to extend the inlet to a location with dryer air than what may be present around the generator.
2) Use a desiccant on the air inlet to remove moisture. We sell a small air dryer cartridge for use with small ozone generators like you have https://www.oxidationtech.com/products/accessories/air-dryer.html. The desiccant is fairly cheap and available on Amazon. I have seen some ozone generators with a smaller cartridge built in. The problem is, they eventually get saturated and need to be replaced or dried out. With a Jacuzzi install, including a tube with a couple gallons of the desiccant beads somewhere in the inlet tube, and extend the inlet tube to the driest location practical with a filter on the end would likely solve any ozone generator problems for the life of the Jacuzzi.
The ozone industry standard is to dry any ozone generator feed gas to a dewpoint of -100 deg F.
For a small 4 gram/hour ozone generator such as the VMUS-4 using dry air as a feed gas, a desiccant air dryer such as the VMD-8 Air dryer https://www.oxidationtech.com/products/accessories/air-dryer/vmd-8.html will get the air down to -40 F dewpoint. This is acceptable for most small air feed ozone applications. It takes years of continuous operation for any nitric acid accumulation to be a problem as long as the air dryer is operating correctly and maintained.
The majority of ozone applications use oxygen as a feed gas. The process used to concentrate oxygen also removes water, and there is very little nitrogen available, so using oxygen largely prevents any nitric acid formation. Oxygen concentrators are often used for ozone generator feed gas https://www.oxidationtech.com/products/oxygen-generator/packaged-units.html and provided clean dry oxygen that prevents nitric acid buildup.
Disposing of nitric acid by regular cleaning or draining it into the ozonated water is to be avoided if at all possible by making provision to dry any feed gas going into an ozone generator. The methods for getting dry air can be as simple and low cost as pulling air from a dry location or using desiccant or more expensive with dehumidifyers and oxygen concentrators. Every bit of nitric acid made by your ozone generator reduces its performance and life span.


Could Nitric Acid build up in pipework?
I inject ozone via a venturi into a foam fractionator, and have found a sticky brown/red residue inside PVC pipe. I’m wondering if this could be nitric acid?
Nitric acid can build-up in a pipe.
However, what you are likely seeing is iron and other minerals precipitating out of water in the pipe after ozone oxidation of those minerals in the water.