We recently posted an article about the decrease of dissolved ozone concentration in spraying water. Find that post here:
https://www.oxidationtech.com/blog/?p=3233
We have always known and operated with the fact that ozone levels in water decrease through the process of spraying water. However, there has been limited research on this phenomenon. We are excited with this research and want to share as much as we can. Below are samples from that paper for easy review. Or, review full research here:
https://www.oxidationtech.com/Downloads/ozone-water/Dissolved-ozone-spray-research.pdf
Authors:
Axel Canado, Marine Tournois, Marielle Pages, Michel Roustan, Wilfried Remus-Borel, Nicolas Dietrich, Frédéric Violleau, Gilles Hébrard
Abstract
Ozone is known to be a powerful oxidant with low persistence and has been shown to possess
biocidal activity against a wide number of fungi and bacteria. These properties make ozone a good
candidate as a substitute for chemical phytosanitary products in agriculture. However, when water
containing dissolved ozone is sprayed, the concentration of ozone decreases dramatically with the
spraying distance. This study aims to better understand this ozone loss during spraying and, in
particular to identify whether mass transfer occurs. Identification was performed by dioxygen
absorption and comparison of its concentration profiles with those for ozone desorption. Droplets
were collected at different heights in the spray to measure the dissolved concentrations. Image
analysis was used to determine the diameters and velocities of the droplets. Ozone self-
decomposition kinetics were not able to explain the experimental concentration variation obtained
in the droplets. The decrease of dissolved ozone in the liquid is then only caused by mass transfer.
Most of the mass transfer seems to take place in the liquid sheet that leaves the nozzle. Volumetric2
mass transfer coefficients have been assessed for different regions of the spray. From mass
transfer correlations, a model has been made to predict the concentration profile in droplets of the
spray.
Introduction
The main goal of the present study was to provide a better understanding of the loss of dissolved13
ozone in droplets during spraying. Solute quantifications along the spray allowed a distinction to be14
made between an ozone mass transfer phenomenon and its self-decomposition. Thus, overall15
characterizations were performed at different distances from the atomizer to track the16
concentration of solute dissolved in the spray. From the measurements, the volumetric mass17
transfer coefficient was assessed for different regions of the spray (Film region and droplets18
region) to highlight the part of the spray where transfer was the most noteworthy. From image19
analysis, the interfacial area of each region has been assessed, then the corresponding has20
also been estimated. A model able to describe the concentration profile of dissolved ozone along21
the spraying distance has been built. This paper may also provide a way to encourage sellers of22
OW sprayers to exercise caution when claiming to reproduce the in vitro effect of OW with spraying23
techniques
Results
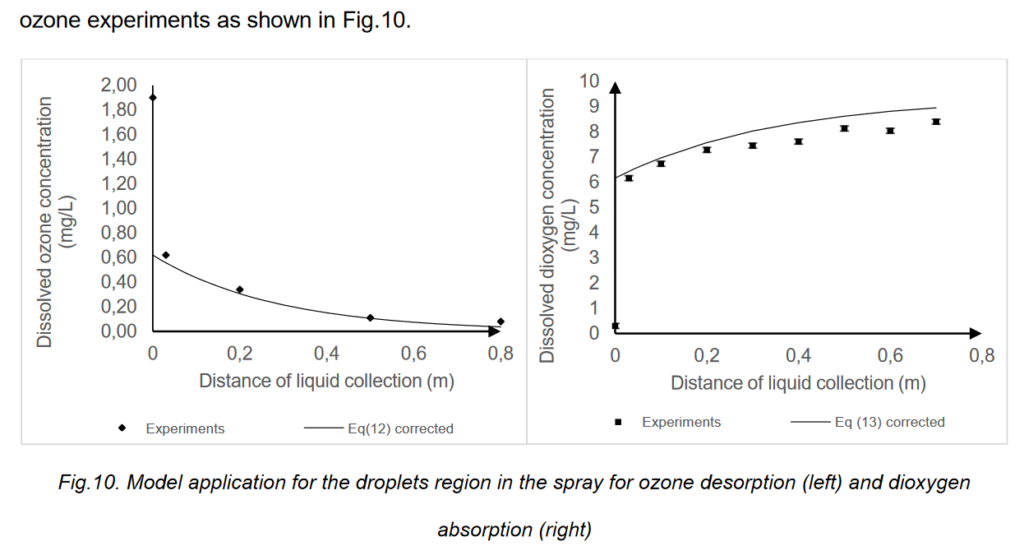
Conclusions
An experimental study has been conducted to identify the phenomenon that causes the loss of2
dissolved ozone in droplets when ozonated water is sprayed. Mass transfer between liquid droplets3
and the ambient air is responsible for these ozone losses. It occurs because the ozone dissolved in4
the droplets is much more concentrated than the saturation concentration in air, which it is naturally5
low. The phenomenon has been identified on the one hand, through performing desorption of6
ozonated water by spraying an ozone-rich liquid in ambient air and, on the other hand, by7
assessing the absorption of dioxygen from air in dioxygen-free water. Concentration profiles have8
been compared and show a significantly similar trend: about 60% to 70% of the transfer is9
achieved within the first few centimetres after the outlet of the nozzle. We have shown that ozone10
self-decomposition kinetics were not able to explain the experimental concentration variation11
obtained into the droplets. The decrease of dissolved ozone in the liquid is then only caused by12
mass transfer phenomenon.13
From mass balances and mass transfer theories, a model has been constructed to predict the14
dissolved concentration along the spraying distance. The calculation requires the droplet diameters15
and velocities in the system to be known. These hydrodynamic parameters were determined by16
particle image analysis, and averaged values have proved suitable to provide an accurate fit with17
the experimental data. The model exhibits discrepancies in the centimetres of the spray near the18
nozzle, which can be explained by the presence of a liquid film that is not yet atomized into19
droplets in this region. Thus, the model considering spherical droplets cannot be applied. Then, the20
model has been applied to the droplets part of the spray and provide a reasonable prediction of the21
solute variation. Further study of the liquid film would allow the mass transfer in this region to be22
predicted. Interface sizing and local measurements of the solute in the liquid film would confirm if23
the high mass transfer reached there is due to surface enhancement and/or improvement in the24
hydrodynamics conditions.
