The Earth’s atmosphere is made up of many layers and ozone is a part of that atmosphere that protects the Earth from the effects of UV rays. Ozone is in the stratosphere layer of the atmosphere. The ozone in the stratosphere absorbs UV rays and other solar radiations. Strong UV rays and other radiations can cause skin cancer, cataracts, and even a suppressed immune system. The stratosphere, where the ozone molecules are located, is about 10-40kms (6-25miles) above the surface of the Earth.
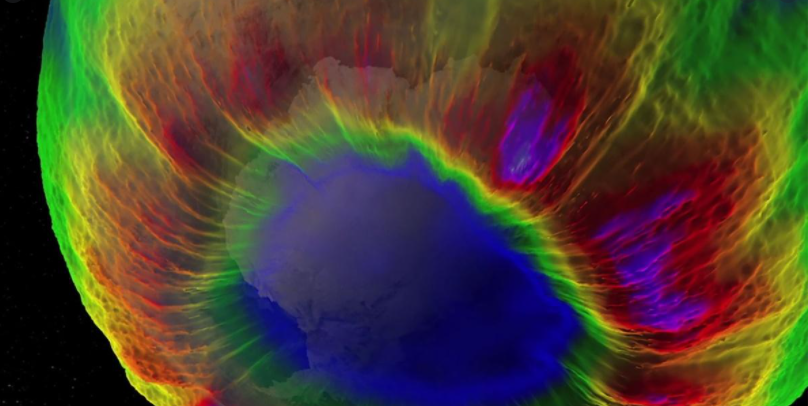
Discovered by British scientists in 1985 Joesph Farman, Brian Gardiner, and Jonathan Shanklin of the British Antarctic Survey, the ozone hole is actually a reduction in concentrations of ozone high above the earth in the stratosphere. Over the past couple of decades, the ozone hole has steadily grown both in size and in the length of existence. Chloroflourobcarbons (CFCs), which are man-made chlorines, are responsible for the thinning of the ozone layer. The ozone layer thinning means there are larger quantities of harmful ultraviolet rays reaching the Earth’s surface. People around the world are starting to make a conscious effort to not use anything that will produce compounds that deplete the ozone layer.
Early Morning Ozone
Ground-level formation of ozone starts early in the morning, and as the day goes on the ozone generation increases, causing pollution. Performing everyday tasks from driving a car to mowing the lawn generates ozone. Volatile organic compounds and nitrogen oxides are the most significant contributors.
Traffic is the biggest contributor to pollution in the air. Continued traffic pollutions added to the earlier ones begins to form ozone as the UV rays intensify. During warm summer months, an inversion may develop and traps the pollutants close to the ground (400-500ft above sea level). Any wind will restrict the pollution dispersion. The UV rays from the sun cause a chemical reaction between the volatile organic compounds and nitrogen oxides to form ozone in the atmosphere. For maximum ozone concentration, temperatures in the 90s are required. The intensity of the UV rays impacts the levels of ozone. The more intense the UV rays, the higher the levels of ozone and the lower the intensity of the UV rays, the lower the levels of ozone. By the evening, most of the ozone that was formed during the day breaks down into other different compounds, and soon enough all of the ground-level ozone is essentially destroyed.
Ozone During Thunder Storms
Everyone has experienced the refreshing feeling after a thunderstorm. This freshness is associated with the formation of ozone. During thunderstorms, the lightning that is produced can conduct more than 25,000 volts. This high voltage causes oxygen in the air to break and recombine to form ozone. The ozone spreads and purifies the air. The rain in the thunderstorm takes away some of the pollutants in the air, as well as contains small amounts of ozone dissolved within the water. This is why we feel very refreshed after and during thunderstorms.
Ozone Formed in Photo Copying Machines, Televisions, X-rays Machines
Anywhere there is high voltage, there is also small amounts of ozone being formed. This is why you can smell ozone behind televisions, in X-ray machines, and in photocopy shops. Ozone is okay to breathe in when it is in small amounts, but doing so continuously can be dangerous. Because of this, it is important to take care of ambient ozone levels. Anyone who works with high voltage producing equipment needs to be aware of the danger of ozone.
Ozone in Refrigerators and Cars
Ozone is used in both refrigerators and cars to purify the ambient air. By doing this, it can ensure longer life for vegetables and other foods inside the refrigerator. High value cars such as Mercedes have small ozone generators systems (in some models) that produces controlled ozone inside the car. This keeps the car fresh from contamination and odor, as odor is the most common problem in air-conditioned cars.