Oxidation Reduction Potential (ORP) is a method to measure oxidation in water. ORP is a common measurement of water quality in a wide variety of water treatment applications. So, what is ORP and how does it work?
Fundamentals of ORP Measurement in water
ORP stands for oxidation-reduction potential, this is a measure, in millivolts of the tendency of water to oxidize or reduce substances within that water or that the water comes in contact with.
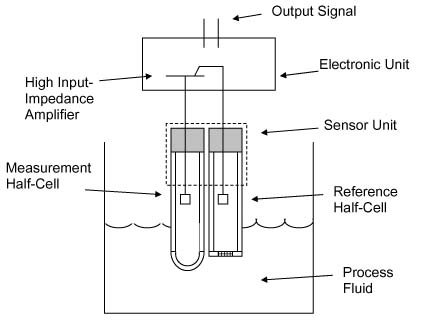
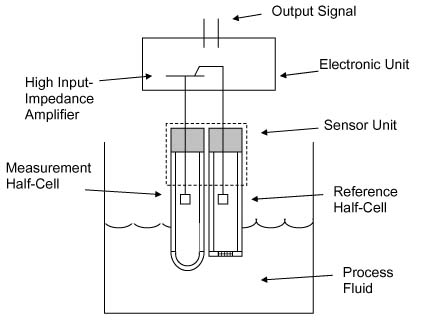
Oxidation is the loss of electrons, or an increase in oxidation state by a molecule, atom or ion. When a substance has been oxidized it’s oxidation state increases. Greater ORP values in water equate to create potentials for oxidation by that water. Any compounds within the water that increase the ORP of the water will be indicated by the ORP meter. In an ozone system, an elevated ORP value can be primarily attributed to the increase of ozone in water along with the resulting ozone by-products, oxygen, hydrogen peroxide, hydroxyl radical, etc.
An ORP sensor in water will measure very small voltages generated by the construction of that probe placed in water. The ORP sensor consists of an ORP electrode and a reference electrode. The principle behind the ORP measurement is the use of an inert metal electrode (commonly platinum) which, due to its low resistance, will give up electrons to an oxidant or accept electrons from a reductant. The ORP electrode will continue to accept or give up electrons until it develops a potential, due to the build-up of charge, which is equal to the ORP of the solution.
ORP sensors will, over time, become less accurate, or cease to provide any voltage changes or usable ORP values. Contamination in the water can coat the ORP sensor probes and cause less accurate ORP readings and slower reactions to changes in ORP values in water. The electrode used in the ORP sensor will also age, due to normal wear of the platinum electrode due to water flowing past the electrode. Due to normal aging and coating of the sensor an ORP sensor should be replaced annually, or as needed to provide accurate ORP readings in water.
Measure ozone in water with ORP
To use the ORP measurement to measure ozone in water a correlation must be made. ORP is a mV signal while ozone is a PPM signal. If the only oxidant added to the water is ozone, a safe assumption can be made that any changes to ORP (mV) are changes in ozone in water. The ozone level in the water can be measured with a manual ozone test kit or meter. This ppm level can be correlated to the mV reading to provide an accurate ozone reading with ORP.

As ORP levels go up the ORP sensor is less and less responsive. To measure ozone in water at levels above 1.0 ppm the ORP sensor will not be suitable.
Why use ORP to measure ozone?
Any time the water is discolored or dirty a dissolved ozone meter will not accurately measure ozone in water. In these cases, ORP is your only option. Also, in most of these applications, dissolved ozone levels are very low, therefore ORP is a valuable method of measuring ozone in water.

I have a hanna instrument that measures ORP, temp and pH and mV and PH simultaneously, how can I measure the Ozone level and what should be the ozone reading for drinking water?
Measure ozone in water with a manual ozone test kit. Links below give options to a few ozone test kits we offer. Correlate this reading with the manual ozone test kit to the ORP value. Do this for a few ORP values and record this. Then, use your ORP meter to measure (indirectly) the level of ozone in water.
https://www.oxidationtech.com/products/ozone-monitors/dissolved-meters/k-7404.html
https://www.oxidationtech.com/products/ozone-monitors/dissolved-meters/i-2022.html
https://www.oxidationtech.com/products/ozone-monitors/dissolved-meters/i-2019.html
In drinking water you should achieve a residual ozone level of at least 0.15 ppm, but maybe much higher depending upon goals and water quality.
I used the millivolt setting on a Hanna H8424 with a Welchem ORP probe.
I found that the Hanna has a hi impedance pre-amp and reads roughly twice the ORP of a multimeter on the mV range.
With a 10 meg ohm resistor across the input the reading from the Hanna is more or less true.
Hope this helps
We are a group of volunteers and opening a new scheme in our community. Your web site provided us with valuable info to work on. You have done an impressive job and our entire community will be grateful to you.
I work for a small algae farm and we use an ozonator to disinfect our RO water. Which values should we aspire to reach to ensure sterile water? Should we wait after the zonation before using the water for the generation of cultivation media? if yes – how long?
Best regards,
Dan
Ideally, you would confirm ozone levels in water with a simple dissolved ozone test kit. Achieve at least 0.5 ppm of ozone in water to ensure sterilization.
Wait at least one hour before using this water in your cultivation. Or, test ozone levels and ensure ozone levels are below 0.1 ppm with a test kit.
The K-7404 test kit would be a great option for this, see details below on this.
https://www.oxidationtech.com/products/ozone-monitors/dissolved-meters/k-7404.html
I am working on a project to oxidize a organic material using ozone water. What is the maximum obtainable ppm one could achieve in a venturi charged closed loop system? I would like to create high amounts of ozonated water using a 7 g/hr o3 generator. I need to “gas” 25 gallons at a time.
The maximum level of ozone in a closed loop is greater than 200 ppm. See link below for some great images of ozone water at levels above 100 ppm and the amazing visual color this creates in water.
https://www.oxidationtech.com/blog/the-color-of-ozone/
In your conditions of 25 gallons of water treated with 7 g/hr ozone your max dissolved ozone level is likely going to be 10 ppm or less due to the limits of ozone solubility. However, I would need more info on your ozone concentration and water temperature to provide a more comprehensive answer.
Ozone levels in water are limited by the solubility of ozone. See link below to learn more about ozone solubility and the constraining factors you are dealing with:
https://www.oxidationtech.com/ozone/solubility.html
We are installing a Ozonation System for sugar molasses. We would like to measure the residual ozone in sugar molasses, after ozonation.
The expected residual concentration is 1 – 3 ppm. Can we use ORP based measurement? How suitable is you K-7404 kit?
Do you know of an equation used to correlate ORP milli-volt to PPM 03 in water?
The water is very controlled, Water For Injection (WFI) level, temp, and pressure levels are very consistent.
4 – 5ppm, 20psi, 15gpm, at 75F temp
Any data available would be great
It is not possible to correlate ORP to ppm o3 with a calculation. ORP measures any oxidant in water including dissolved oxygen. Baseline ORP can vary from 200 – 450 depending upon dissolved oxygen, chlorine and other oxidiants in water. You are looking for an increase in ORP from baseline to measure ozone. The actual value is relative to the start-point.
ORP is also a poor tool to measure ozone in water above about 2 ppm. The ORP value will change only slightly from 2 – 5 ppm. If you are measuring ozone at 4-5 ppm in water, I would suggest using an actual dissolved ozone sensor vs ORP.
What is the safe levels for a 3x2m swim spa. Two of my grandsons develop a rash from being in the water.
Can you recommend testing kits
Thank you
Leonie
If you are looking for a great ozone test kit to measure ozone in water, the K-7404 is my preferred device:
https://www.oxidationtech.com/products/ozone-monitors/dissolved-meters/k-7404.html