Nuts About Nanobubbles
Nuts about Nanobubbles
Nanobubbles are all the rage these days. We are talking here about bubbles even smaller than the micro bubbles that form a milky cloud. These bubbles enter the microscopic realm where they are able to do things that bigger bubbles can’t do. Nanobubbles would be the microbial beach ball. They are so small that they don’t float to the surface like larger bubbles, and can remain suspended in water indefinitely. Undoubtedly these 70-120 nanometer bubbles have unique properties that open new frontiers in water treatment. Are nanobubbles in a position to topple the 100 year reign of ozone in water treatment, or are they conquering territory where ozone is powerless? Perhaps as a joint force, ozone nanobubbles will work together to conquer the ever increasing tide of water problems we face today.
Moleaer https://www.moleaer.com/ has been a pioneer in developing technology to generate these nanobubbles, and their website promotes a wide range of applications which often appear to overlap the applications of ozone. If I have an application where nanobubbles and ozone both claim to provide a solution, which would be the best option? Ozone is a relatively old technology backed by a hundred years of tried and true application, and libraries of research and data. Nanobubbles are a young emerging technology driven by new studies and exciting stories of successful application to problems. Our goal at Oxidation Technologies is to make sure you are empowered to make an informed choice.
When nanobubbles and ozone are both stripped down to their fundamental task, we see that nanobubbles and ozone both serve as a vehicle to deliver energy into the water where it can do something useful. Energy to fight off pathogens. Energy to boost plant growth. Energy to break down contaminants. Electrical energy turns the motors that compresses the air and pushes the water to slice up and squeeze out the very tiny bubbles. Electrical energy turns the motors to concentrate oxygen and pulls the O2 molecules apart in a plasma cell to make O3. In both cases, energy is packaged in such a way that it can be delivered and released again to super saturate water with oxygen, or smash microscopic and molecular targets. Like a tightly wound spring, a nanobubble carries this energy as a very tiny high energy bubble. Ozone caries this energy in a high energy molecular structure.
Wherever you look, energy is needed to clean things up. The sun supplies energy to drive the water cycle and naturally clean up the toxic byproducts of life. Water treatment plants replicate this process with large pumps, chemical application, and filters – all consuming more energy. In this respect, both nanobubbles and ozone require a considerable input of energy.
In addition to understanding nanobubbles and ozone as energy vehicles, it is important to understand that an ozone molecule is much smaller than a nanobubble. A 127.8 nm nanobubble has a diameter equivalent to 1000 ozone molecules. Ozone gas molecules can ride in a nanobubble like cars on a ferry. While riding a ferry can be useful, generally cars are more useful when they are not on a ferry. The same applies to the relationship between ozone and nanobubbles. Is it really helpful to have ozone in a nanobubble? We will explore this shortly. The point I want to make here is that nanobubbles float around in water, while ozone joins the water on a molecular level to do its work.
The idea of ozone in a nanobubble sounds like the ultimate silver bullet, but making an ozone nanobubble is not as easy as it sounds, and the result is anticlimactic. When a customer calls looking for an ozone generator to use with a nanobubbler, the first question I need to ask is “how much pressure do you need for your nanobubbler to work?” When it comes to nanobubble generatoars, in general more pressure is better – ranging from 15 psi to 120 psi. I need to explain that ozone is generated most easily at lower pressures. High pressure ozone generators are available … they just cost more. Then I also need higher pressure oxygen for a feed gas. An ozone compressor is an option, but those are high maintenance, costly, and prone to leaks. So the question needs to be answered, is my goal really worth the extra cost and energy needed to get ozone inside the nanobubble?
Suppose you invest in a high pressure ozone generator and high pressure oxygen source and manage to get ozone inside the nannobubbles. Henry’s Law drives the ozone quickly out of the nanobubble and into solution where it just as quickly leaps into its suicide mission to engage the nearest oxidizable molecule and it is all over. The nanobubble continues on floating through the water while Henry’s Law pulls the remaining oxygen molecules from the bubble until an equilibrium is established. The question is, Was this really worth all the effort of getting ozone into the nanobubble?
If the goal is to dissolve ozone and oxygen into water, a low pressure ozone generator, water pump and venturi will do that very well. The venturi will even make some nanobubbles with a good stout pump and a good quality venturi. It does not require any patented plumbing either, just an understanding of basic pressure, flow and solubility laws. Getting 90% of the ozone into solution this way ranks right up there with the nanobubble score.
If your goal is to get oxygen dissolved into water, the a nanobubble machine is a more attractive option. But even here, the need for high pressure oxygen to feed the nanobubbler should be weighed against lower pressure oxygen to feed a pump and venturi injection skid. I have yet to see a neck to neck performance comparison between a nanobubbler setup and a venturi skid. I would be interested to know the 10 year cost of maintaining a set dissolved oxygen level in a body of water using both methods.
If you goal is to remove pathogens or oxidize other materials in the water, ozone has proven to be an effective solution. Packing a 2.07 volt electrochemical potential, ozone ranks well above Chlorine and Hydrogen Peroxide. This makes ozone a fantastic choice for destroying microscopic pathogens. But nanobubbles claim, under special conditions, to collapse and form the Hydroxyl Radical which boasts a 2.8 volt electrochemical potential. We await to know what is needed to create these special conditions and how much Hydroxyl Radical is produced.
I look forward to seeing where and how nanobubble technology takes hold to solve. If you have a shallow stagnant pond, or you need to separate suspended particles nanobubbles do a great job.
I also have questions about using nanobubbles to get oxygen in water. I don’t know if I agree with the following statement: “Dissolved oxygen is a measure of how much oxygen is present within a body of water.” When nanobubbles persist for a long time in water as nanobubbles and accumulate over time, does that count as oxygen present in water? If so, it is not yet dissolved, so it should not be included as a measure of dissolved oxygen. Are nanobubbles taken up as oxygen into a fishes gills or participate in the oxidation of dissolved iron, or do the nanobubbles serve more as time release capsules that replenish dissolved oxygen levels as they are depleted?
I also have many questions
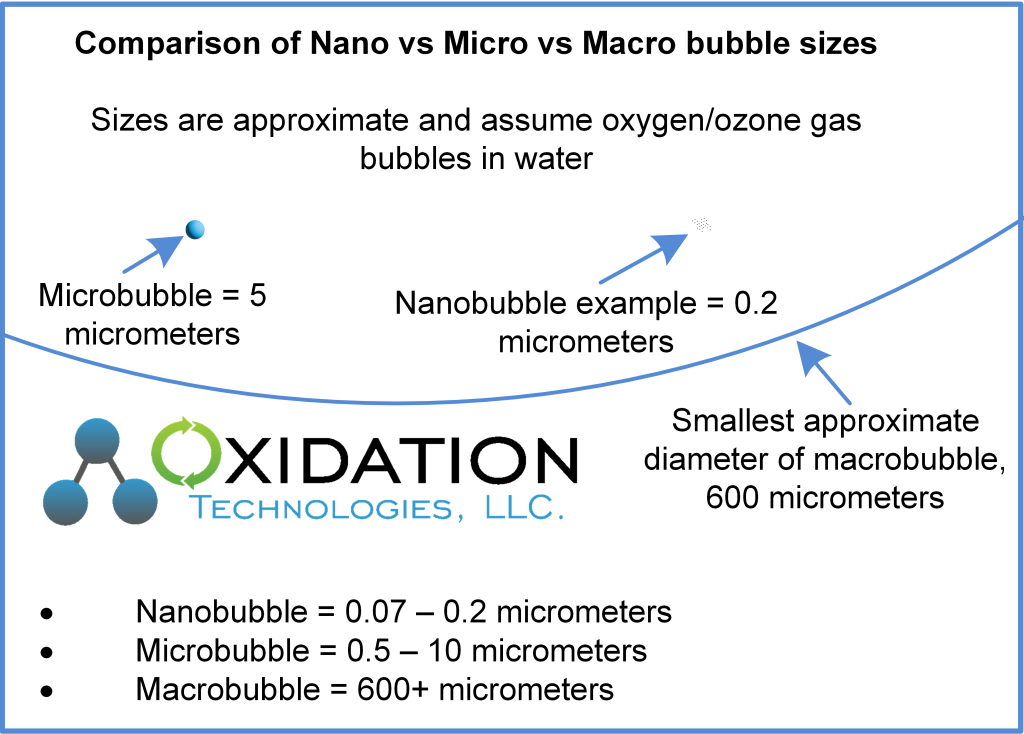
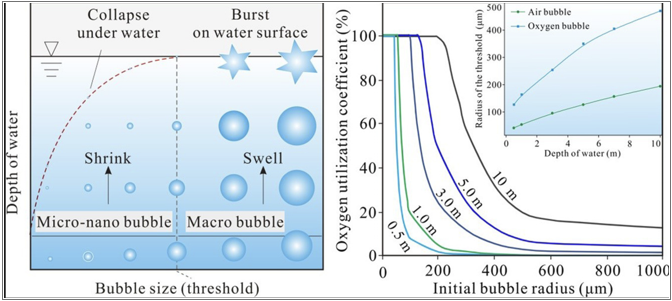
Is there an optimal bubble size for mass transfer? If the bubble is too big, it holds a large volume of oxygen, has a low surface area to volume ratio, and floats to the surface quickly – all factors that limit the mass transfer of oxygen from gas to solution. If the bubble is smaller, it floats upwards more slowly and more of the oxygen contained in the bubble gets dissolved. Clearly smaller is better, but is there a point of diminishing returns? How long do you need a bubble to last? How long does it take for oxygen to move from gas to solution? Henrys Law factors in pressure and temperature. So what is the benefit of a bubble that stays suspended long after all the oxygen has entered solution?
The following graphic from Science Direct is helpful for some of these questions. It clearly shows that nanobubbles are especially suitable in a very shallow water situation where the only option is. Water depth increases pressure and also the time. A well designed injection skid incorporates this pressure and time, and illustrates that even macrobubbles are 100% utilized.
In conclusion, I question the following claims of nanobubble technology:
Enhanced oxygen solubility – “superior to all other scalable gas transfer methods.” This may be true if compared to bubble diffusers, but not a venturi or even static mixer under pressure.
Ozone application – “enabling customers to apply more ozone, a known oxidizing gas, with greater transfer efficiency.” This claim comes with no evidence or documentation. Since ozone is 16 times more soluble than oxygen, and it’s half life is rather short, I question the need to use long lasting nanobubbles to increase transfer efficiency.
Here at Oxidation Technologies we are particularly interested in getting ozone dissolved into water. When ozone gas is dissolved into water, the individual gas molecules are joined to the water molecules where they can oxidize even the smallest pathogen and oxidize Our current water ozone injection skid is designed to generate ozone and dissolve 90% of the ozone into water as it enters and exits the skid.
Additional Informaton about Nanobubbles
Oxygen generators can be found HERE
Ozone Solubility information HERE