Nanobubbles
Ozone Nanobubbles are tiny gas bubbles measuring less than 0.0002 millimeters in diameter, mostly invisible to the naked eye. Unlike normal bubbles in water nanobubbles do not rise in water due to buoyancy but remain in water flowing with the current of that water. Nanobubbles can retain in the gas phase, bubble form long-term and may be fairly stable in water.
Ozone Nanobubbles are used to aid and improve dissolution of ozone gas into water. As ozone is only partially soluble into water mechanical means are necessary to dissolve ozone gas into water efficiently. Nanobubble creation is one method of improving ozone mass transfer into water.
Lean more about ozone solubility into water HERE
What is a Nanobubble?
How small is a nanobble? What qualifies as a nanobubble?
The concept of Nanobubbles, sometimes referred to as ultrafine bubbles was introduced to describe the unique physical properties and action of these bubbles rather than the physical size. Nanobubbles are tiny gas bubbles in liquid, since various gasses in various liquids will create the unique properties of a nanobubble in different sizes of bubbles. Therefore, the size of the bubble is less of a part of the definition as the properties and action of the bubble.
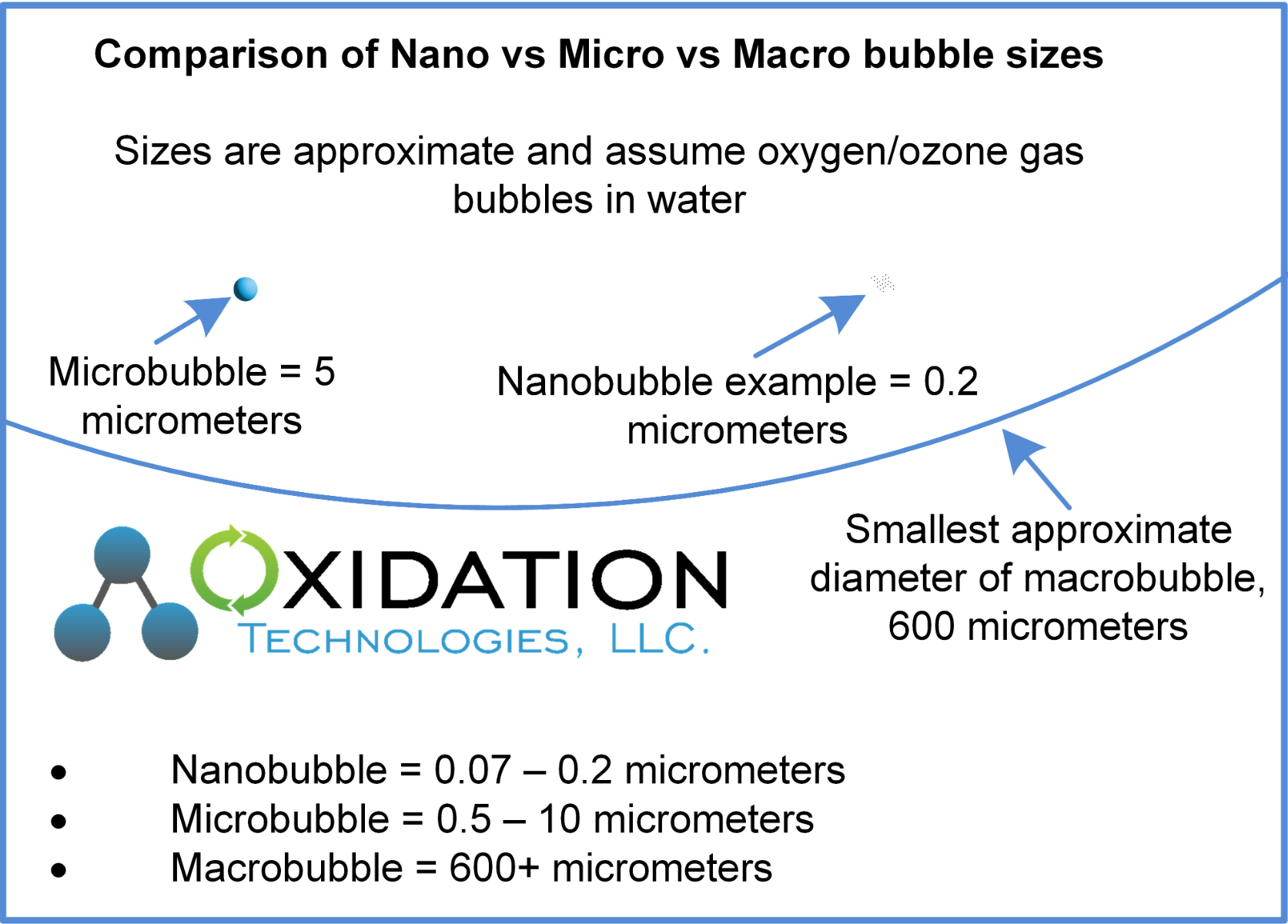
For our purposes, we are referring to the ozone/oxygen gas bubble within water. In this case, the size of nano vs micro vs macro bubbles are categorized as follows:
Nanobubble = 0.07 – 0.2 µm (micrometers)
Microbubble = 0.5 – 10 µm (micrometers)
Macrobubble = 600+ µm (micrometers)
Unique Properties of Nanobubbles
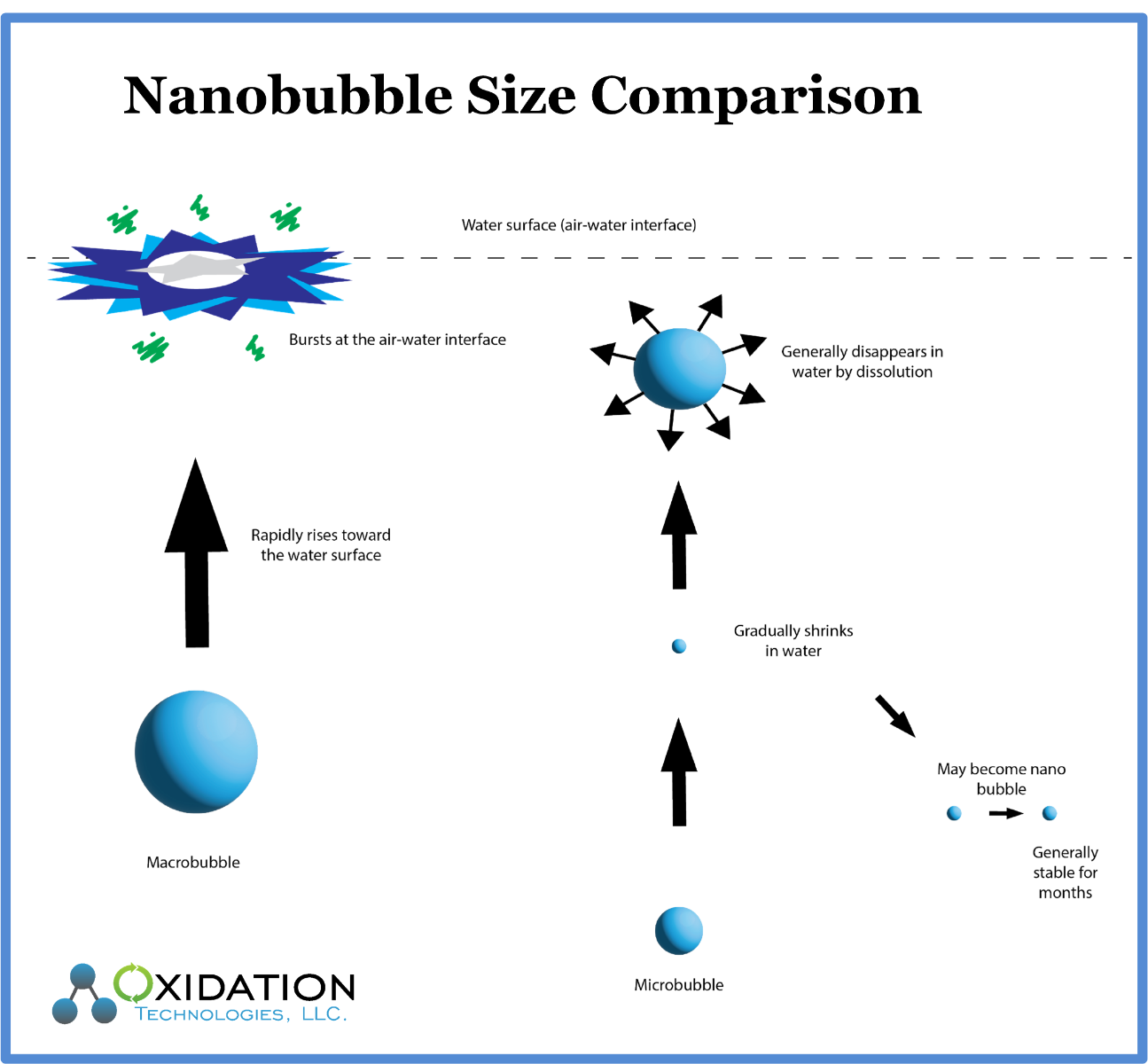
Nanobubbles are defines by their unique properties in water. These unique properties may be beneficial in dissolving ozone/oxygen gasses into some waters. In addition, after ozone has reverted to oxygen due to natural half-life decay, the remaining oxygen Nanobubble may continue to dissolve into water, and oxidize compounds in water for long periods of time. There are reports of Nanobubbles remaining in water for multiple weeks.
Unlike Macrobubbles, Nanobubbles do not rise to the surface of the water due to buoyancy. Unlike Microbubbles, Nanobubbles do not disappear in water by dissolving into the water but remain in the nanobubble (ultrafine) bubble state. The nanobubble with a long lifetime in liquid move around randomly rather than upward as larger bubbles would. This random, sometimes lateral, sometimes vertical, even downward phenomenon is referred to as the Brownian motion.
Nanobubbles in water have a strong negative charge, sometimes referred to as a negative Zeta Potential. This negative charge to the nanobubbles can be measured in mv and can be used to quantify nanobubbles in water.
Free Radical Creation
Nanobubbles in water have a strong negative charge that can and will react with water to create Hydroxyl Radicals (-OH). This phenomenon is more prevalent with oxygen nanobubbles, and even more prevalent with ozone nanobubbles. Sometimes referred to as Advanced Oxidation Processes (AOP), the resulting Hydroxyl radical is a more powerful oxidant than ozone alone.
The strong negative charge and Zeta Potential of the nanobubble causes the interaction of the oxygen within the nanobubble to react with the water to create Hydroxyl Radicals with no other outside energy required.
The Ozone Nanobubble advantage
The Ozone Nanobubble has specific and unique advantages. While Nanobubbles can be created with many gasses in liquid, our focus is the Ozone, andOxygen Nanobubbles.
Half-life of ozone improvements
The ozone molecule is inherently unstable and will revert back to oxygen through natural half-life in a short period of time. Therefore, there is no method to store ozone gas, or ozone in water. Using Nanobubble technology the half-life of ozone in water can be extended.
The half-life of ozone in the gas phase is higher than the half-life in aqueous phase. This property allows the ozone gas within the Nanobubble in water to remain in the ozone vs oxygen state, while the ozone in water either reacts with oxidizable compounds and pathogens or decays naturally due to half-life. This ozone gas contained in the nanobubble can then dissolve into water as the ozone/oxygen saturation point allows for additional gas dissolution into water. This will cause the ozone levels in water to remain higher for longer periods of time.
In the paper, Disinfection application of micro and nanobubbles, a reference is made to an ozone level in water that is extended 1.6x when micro and Nanobubbles are implemented vs macrobubbles alone.
Improved Dissolved Oxygen Levels
Ozone has a higher solubility rate than oxygen into water. It dissolves more rapidly into water than oxygen, and when it decays back to oxygen, it creates higher dissolved oxygen levels in water than what might be possible when oxygen alone is dissolved into water. When Nanobubble technology is also employed, the half-life improvements of ozone in water can be leveraged to dissolve ozone into water as oxygen in water is consumed by outside factors in applications such as aquaculture and hydroponics.
Hydroxyl Radical creation (AOP)
Referenced above in free radical creation, Nanobubbles containing oxygen have the potential to create Hydrol Radicals (-OH) in water. Ozone is used in the AOP reaction with either UV-Light or Hydrogen Peroxide as the catalysts to create Hydroxyl Radicals in water. Research has proven that ozone alone can create high levels of Hydroxyl Radicals in water when Nanobubble technology is employed.
Antimicrobial action benefits
Ozone gas and ozone dissolved into water are excellent disinfectants. The ability for ozone to inactivate bacteria and viruses is widely known. However, there are applications where the addition of nanobubble technology improves this antimicrobial potential. Due to the strong negative charge associated with a nanobubble (referred to as Zeta Potential) positively charged bacteria can be attracted to the nanobubble containing ozone allowing faster and more efficient antimicrobial action.
Gas Solubility Advantages
Nanobubble technology can improve gas transfer into liquid, or solubility of gasses into liquids. While nanobubbles are normally thought of as long-lasting tiny bubbles in water this phenomenon is most common when saturation of gasses into liquids is achieved. Prior to saturation of gas into liquid improvements in solubility, or mass transfer are achieved any time smaller gas bubbles are used to dissolve gas into liquid.
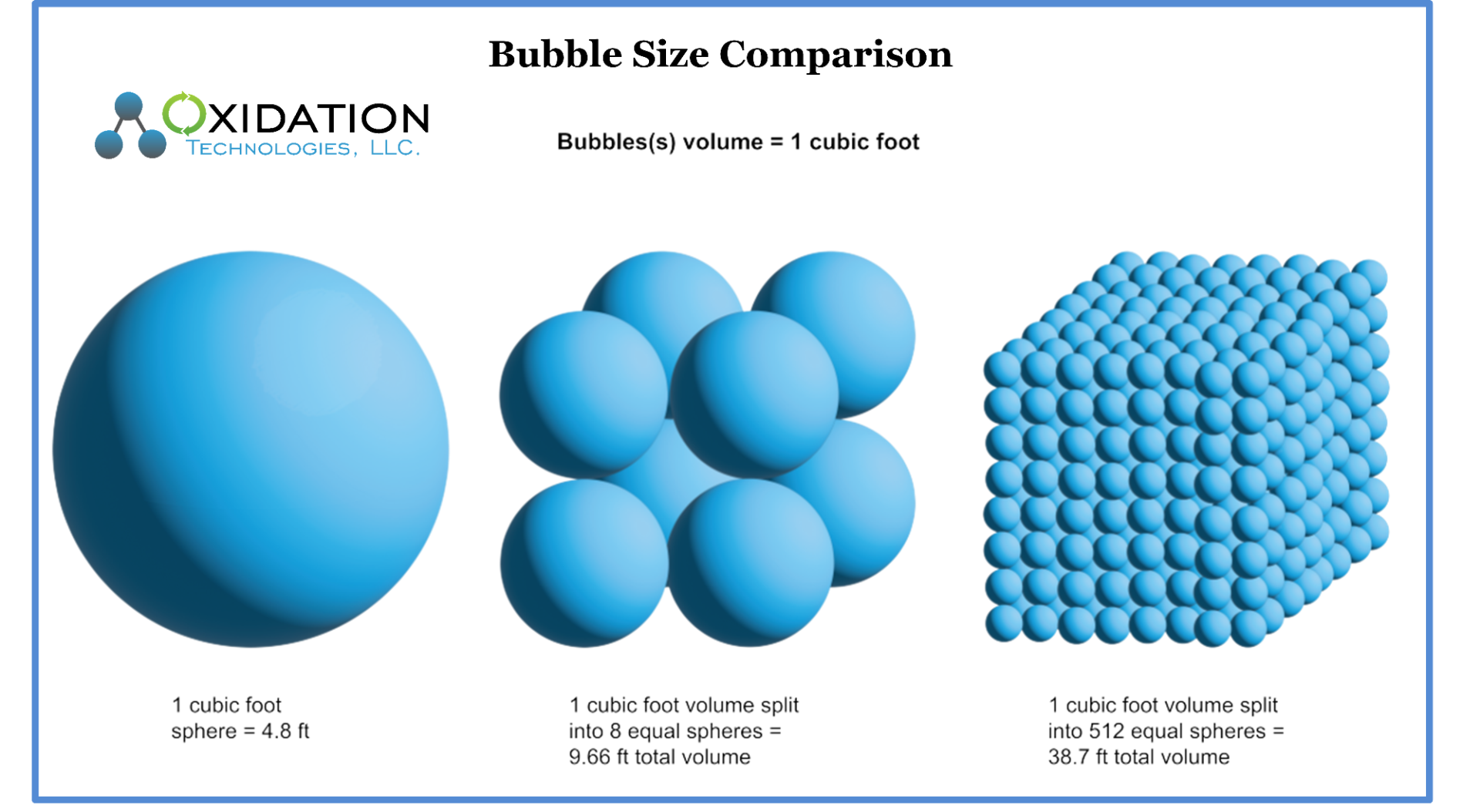
The transfer of gas into liquid is highly dependent upon the interface between the gas bubble and liquid. Due to the higher surface area of the gas in relation to bubble size, smaller bubbles will dissolve into liquid more efficiency than larger bubbles given the same overall volume of gas.
See drawing below for a great example. This drawing illustrates the same 1-cubic foot volume of gas and bubble size in relation to surface area of those resulting bubbles.
Creation of Nanobubbles
Nanobubbles can be generated in water by creating cavities in water, cavitation. Cavitation in water is created by reducing pressures below a critical value that will be application-dependent. Cavitation in water is typically broken down into the following types
- Hydrodynamic - Variations in the pressure of the water
- Acoustic - Produced by applying ultrasound to water
- Optical - Short-pulsed lasers focused into low absorption coefficient
- Particle - Passing high intensity light photons in water
The generation of nanobubbles using any technology is greatly influenced by pressure, temperature, type of gas, concentration of gas, and water quality.
While nanobubbles can be created in water initially using various methods, water must be at saturation point for oxygen/ozone gas to remain in a nanobubble or microbubble state for any period of time. Prior to saturation point the gas will dissolve directly in the water efficiently, while the nanobubble creation may be credited (slightly) for improved solubility of gas, no other benefits are realized from nanobubble technology if saturation point is not reached.
Creating ozone/oxygen nanobubbles in water is almost always performed using hydrodynamic methods. Water is typically getting pumped and ozone/oxygen is being dissolved, (within these systems), nanobubbles can be created simultaneously. The hydrodynamic methods are widely used. The following hydronamic methods can be employed.
- Venturi Injector - operating at high water pressures and excellent suction will create cavitation necessary for nanobubble generation
- Venturi Injector + Static Mixer - operating at high water pressures will achieve saturation of water using the venturi and creating cavitation through shearing factors at the static mixer (this can also flash ozone gas out of water if done improperly)
- Cavitation Pumps - create high suction causing cavitation at the inlet side of the pump, commonly employed with orifices to create necessary suction. Ozone/oxygen can be introduced into the suction side of the cavitation pump to both dissolve gasses and create nanobubbles
- High-Pressure Vortex - will require oxygen/ozone gas to be introduced into the system at high pressures (80 PSI +) prior to a vortex to break the gas into tiny bubbles
- High-Pressure Diffusers - will use tiny holes in a gas diffuser to break the gas into tiny, nano-sized bubbles directly in the water. This will require a high pressure of oxygen/ozone gas for operation.
One challenge nanobubble systems may encounter is energy consumption. Some of these systems require a higher amount of energy than may be warranted in a specific application. If possible, test simpler system designs with lower energy consumption rates versus specific nanobubble creation equipment to determine if the higher energy consumption rate is warranted.
Measuring Nanobubbles in Water
Measuring Ozone or Oxygen Nonobubbles in water may be a necessary step in validating a specific process. As ozone alone is a very effective disinfectant and breaks down into oxygen the benefits seen from a system may be due to ozone oxidation versus any nanobubble creation. The good news is, there are methods of verifying and quantifying nanobubble generation in water along with the specific size of nanobubbles.
- Light scattering technique - Simple method to verify the presence of nanobubbles in clear water using light beams passing through the water. Does not validate size or quantity well.
- Nanoparticle tracking analysis - Dark field microscopy is used to capture the movement of scattering objects. This method can quantify both the quantity and size of nanobubbles due to the microscopy equipment utilized.
- Dynamic light scattering - A laser beam illuminates the sample and fluctuations of the scattered light are detected by a photon detector at a scattering light angle. This system is likely the most commonly used and will measure both size and quantity of the nanobubbles.
- Zeta potential - Due to the strong electron affinity of the nanobubble causing an elevated Zeta Potential in the water the prevalence of nanobubbles in water can be validated by measuring Zeta Potential in water. This will not measure the size of nanobubbles and is generic at best.
There are methods that have been studied or evaluated not mentioned here. To date, most commonly the Dynamic Light Scattering method has been used. As this technology develops additional measuring methods may be implemented that are effective.
Summary
Ozone Nanobubbles have gained a great deal of attention recently. There are great opportunities for ozone nanobubble technology within water treatment and disinfection applications. In fact, the proper implementation of nanobubbles in synergy with ozone may be as effective as traditional AOP technologies at a much lower cost in some applications.
However, ozone dissolved efficiently in water without specific Nonobubble generation technologies is still sufficient and compares identically to Ozone + Nanobubble technology in many applications where ozone dissolved into water is the primary goal and mode of action for water treatment. Therefore, the capitol cost and energy consumption of nanobubble specific technologies should be weighed against the actual requirements of each project carefully.
Additional Ozone Nanobubble Information - "Nuts About Nanobubbles"
White Papers and References Below:
Below is a list of compiled white papers and references regarding the use of ozone nanobble technologies in a wide variety of applications.
Should you have additional questions, or desire other references, please contact our office.
Full access papers links to PDF documents below:
- Disinfection applications of ozone micro- and nanobubbles
- Stability of Nanobubbles
- Are Nanobubbles really a big deal?
- Nanobubble ozonation for waterbody rejuvenation at different locations in India: A holistic and sustainable approach
- Ozone nanobubble treatment in freshwater effectively reduced pathogenic fish bacteria and is safe for Nile tilapia (Oreochromis niloticus)
- Effect of ozone nanobubbles on the microbial ecology of pond water and safety for jade perch (Scortum barcoo)
- Effects of ozone nano-bubble water on periodontopathic bacteria and oral cells - in vitro studies
- Control of Vibrio parahaemolyticus (AHPND strain) and improvement of water quality using nanobubble technology
- Applications of OZone Micro and Nanobubble technologies in water and wastewater treatment: Review
- A remedy for harmful algal blooms? Scientist thinks he's found one
Abstracts/Summaries with links to full documents below:
Mechanism for Enhancing the Ozonation Process of Micro- And Nanobubbles: Bubble Behavior and Interface Reaction
Authors: Xiaolong Yang, Li Chen, Seiichi Oshita, Wenhong Fan, and Shu Liu
Abstract
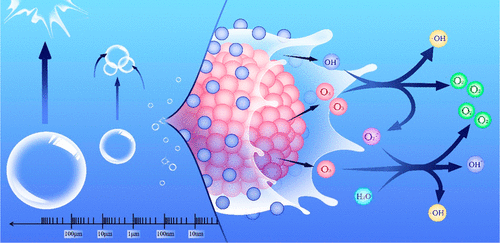
The combination of micro- and nanobubble (MNB) technology with ozonation is a promising wastewater treatment technique that increases the solubility and oxidation capacity of ozone. However, the underlying mechanism remains unclear. We investigated the oxidative capacity of reactive oxygen species (ROS) produced by ozone bubbles of different sizes. Our results show that the process of bubble shrinkage and collapse facilitates the production of ROS. In the domain of MBs with particle sizes ranging from approximately 1 to 100 μm, a reduction in bubble size is positively correlated to increasing tendencies toward bubble shrinkage, collapse, and disappearance. However, ozone NBs have a longer lifespan than MBs, allowing them to collapse continuously and produce ROS over an extended duration of storage time. We studied the specific reaction of ROS production at the gas–liquid interface and confirmed that ozone MNB water produces hydroxyl radicals (•OH) but not hydrogen peroxide (H2O2). •OH is produced by a reaction between ozone and hydroxide ions on the surface of the bubbles. When applied to treating actual reclaimed water, the ozone MNBs shortened the sterilization time of E. coli. The long-term production of •OH by ozone MNBs can be advantageous in water disinfection systems.
https://pubs.acs.org/doi/10.1021/acsestwater.3c00031
Characteristics and Stability of Ozone Nanobubbles in Freshwater Conditions
Authors: Meryem Soyluoglu, Daekyun Kim, and Tanju Karanfil*
Abstract
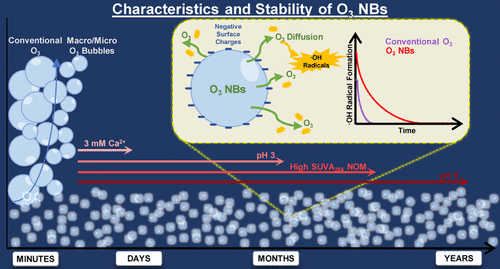
The characteristics and stability of ozone nanobubbles (NBs) were investigated for the first time under different preparation conditions and freshwater conditions (i.e., pH, natural organic matter [NOM], carbonate, calcium, and temperature) for an extended period. Two oxygen gas flow rates (4 and 1 L/min) used in ozone NB generation affected the characteristics and stability of ozone NBs. The ozone NBs generated at a high initial dissolved ozone (12.5 mg/L) concentration showed a much higher brightness during measurements than the ozone NBs generated at a low initial dissolved ozone concentration (1 mg/L). The former also exhibited a higher negative surface charge and higher stability in comparison to the latter. The stability and half-lives of ozone NBs followed the order of 3 mM Ca2+ < pH 3 < NOM with high specific ultraviolet absorbance at 254 nm (SUVA254 = 4.1 L/mg·m) < pH 7 < pH 9, while the effects of carbonate and temperature were insignificant. Ozone NBs were relatively stable in waters for a long period (e.g., ≥ 60 days) except for high hardness or low pH conditions. Higher levels of hydroxyl radicals were produced from ozone NB solutions as compared to conventional zonation.
https://pubs.acs.org/doi/abs/10.1021/acs.est.3c07443
Solubilization and stabilization for prolonged reactivity of ozone using micro-nano bubbles and ozone-saturated solvent: A promising enhancement for ozonation
Authors: Wei Fan, Wen-gang An, Ming-Xin Huo, Wu Yang, Sui-yi Zhu, Shan-Shan Lin
Publisher: Separation and Purification Technology Volume 238, 1 May 2020, 116484
ABSTRACT
The enhanced ozonation treatment by a combination of micro-nano bubbles (MNBs) and ozone-saturated solvent (e.g. acetic acid (HAc)) was investigated. The solubility, decomposition rate of ozone, and reactive oxygen species (ROSs) in various concentrations of HAc with/without MNBs were examined, and these synergetic effects were verified by the enhanced removal of p-chlorophenol (4-CP). The results showed that MNBs promoted the solubility and mass transfer coefficient (KLa) of ozone in both deionized (DI) water and HAc solution by 2.03–2.26 and 2.30–3.68 times respectively compared with normal millibubbles. Addition of a certain amount (0.5–5%) of HAc can further benefit the ozonation process. The ozone solubility was about four times larger in 5% HAc solution compared with that in DI water (0%), while the former KLa was 3.91-fold higher. The average half-lives of ozone were prolonged by 1.39–3.52 times in 0.5–5% HAc solutions to water alone. This was attributed to the inhibited chain reaction by scavenging the hydroxyl radical ( OH) in the presence of HAc and thus retarded
OH) in the presence of HAc and thus retarded  OH-catalyzed ozonolysis. The detection of ROSs showed that the concentrations of hydrogen peroxide (H2O2) and
OH-catalyzed ozonolysis. The detection of ROSs showed that the concentrations of hydrogen peroxide (H2O2) and  OH were up to 39.53 ± 4.10 and 70.17 ± 3.09 μg/L in the hybrid system. The ROSs led to that 48.15–77.55% of 4-CP were removed by ozonation with MNBs in 30 min, and 9.50–29.40 percent improvements were achieved in the HAc solutions than in the DI water. These results indicate that the combination of ozone with MNBs and HAc could enhance the removal of 4-CP effectively. This study illustrated the solubilization and stabilization effect for prolonged reactivity of ozone using MNBs and ozone-saturated solvent together.
OH were up to 39.53 ± 4.10 and 70.17 ± 3.09 μg/L in the hybrid system. The ROSs led to that 48.15–77.55% of 4-CP were removed by ozonation with MNBs in 30 min, and 9.50–29.40 percent improvements were achieved in the HAc solutions than in the DI water. These results indicate that the combination of ozone with MNBs and HAc could enhance the removal of 4-CP effectively. This study illustrated the solubilization and stabilization effect for prolonged reactivity of ozone using MNBs and ozone-saturated solvent together.
Introduction
Ozone, as a powerful oxidant, reacts direct or via an indirect hydroxyl radical ( OH) pathway to decompose organics, improve biodegradability, and inactivate microorganisms in water treatment and on-site remediation [1], [2], [3]. However, more widespread implementation of ozone is limited by its low mass transfer efficiency, low saturation solubility, and short half-life [4], [5]. These limitations often lead to lower reaction concentration and underutilization of ozone in wastewater treatment of ozonation. The half-life for aqueous ozone usually is less than 1 h due to its high reactivity [6], which results in that the effective reaction zone in situ flushing site is likely to be confined at short distances away from the ozone injection point [7], [8]. Therefore, solubilization and stabilization for prolonged reactivity of ozone are of critical importance to many processes in the field of wastewater treatment and on-site remediation.
OH) pathway to decompose organics, improve biodegradability, and inactivate microorganisms in water treatment and on-site remediation [1], [2], [3]. However, more widespread implementation of ozone is limited by its low mass transfer efficiency, low saturation solubility, and short half-life [4], [5]. These limitations often lead to lower reaction concentration and underutilization of ozone in wastewater treatment of ozonation. The half-life for aqueous ozone usually is less than 1 h due to its high reactivity [6], which results in that the effective reaction zone in situ flushing site is likely to be confined at short distances away from the ozone injection point [7], [8]. Therefore, solubilization and stabilization for prolonged reactivity of ozone are of critical importance to many processes in the field of wastewater treatment and on-site remediation.
Micro-nano bubbles (MNBs) are defined as ultrafine bubbles with diameters on the order of micrometers and nanometers. MNBs feature high internal pressures and rapid mass transfer rates due to the small size, which can significantly improve gas dissolution [9]. Compared with normal millibubbles, MNBs show lower rising velocity in the aqueous solution and may persist in water for long periods. Bubbles of radii 150–200 nm have been shown to remain stable for two weeks [10], and bubbles gathered into clusters could further increase their stability [11]. Consequently, MNBs can migrate with the water flow and provide continuous gas supply for the dissolution phase. In recent years, MNBs has become a research hotspot in the field of environment. Zheng et al. found that the ozone mass-transfer coefficient was 2.2 times higher than those of the macrobubble-ozonation [12]. Li et al. reported that the MNB emulsion could remain stable and breakthrough the sand column without obvious reduction of the hydraulic permeability [13]. Therefore, MNBs offer a promising solubilization and stabilization method for ozonation.
In addition to stabilize ozone in the form of ultrafine bubbles, use of ozone stabilizers has been also considered for enhanced ozone dissolution, stabilization in aqueous phase and even transportability within subsurface [14], [15]. The targeted stabilizer should be required to dissolve ozone in high concentration without heavy ozone consumption. Martha et al. revealed that HAc could dissolve more ozone and thus enhanced the remediation of TCE-contaminated soil by flushing with ozone in HAc [16]. Hiragaki et al. generated ozone foam by surfactant to get relatively high dissolving ozone that was about three times larger compared with that estimated using the Henry constant [17]. Dettmer et al. founded that hydroxypropyl-β-cyclodextrin could stabilize aqueous-phase ozone and prolong oxidation potential through inclusion complex formation [18]. Thereinto, HAc was considered as one of suitable stabilizers as it could be naturally decomposed, easily biodegraded, and it often works as adding chemicals or reaction products in site-remediation [19]. It presents non-reactivity with ozone, a high capacity to dissolve ozone and promotion for the formation of  OH by ozone.
OH by ozone.
However, the combination of MNBs and HAc for enhanced ozonation has not been reported so far. It is hypothesized that the saturation concentration, the retention time of aqueous ozone, and the production of oxidative free radicals would be promoted in the hybrid system with MNBs and HAc, thus accelerating the oxidation of ozone to pollutants. The purpose of this study is to investigate the solubility, decomposition rate of ozone, and ROSs in various concentrations of HAc with/without MNBs. Then the enhanced remediation efficiency of this combined method was verified using 4-CP as a representative refractory organic pollutant in water solution. The 4-CP is one type of chlorinated aromatic compound, which is of high toxicity, bioaccumulation and persistence in environment.
Conclusions
The performance of solubilization and stabilization for prolonged reactivity of ozone using catalytic MNBs ozonation with ozone-saturated solvent (HAc) was investigated. The solubility, half-life of ozone, and reactive oxygen species in various concentrations of HAc with/without MNBs were compared. The comparisons showed that MNBs promoted both the solubility and KLa of ozone by 2.03–3.68 times in DI water and HAc solution. Addition of a certain amount (0.5–5%) of HAc with MNBs ozonation was
https://www.sciencedirect.com/science/article/abs/pii/S1383586619353341
New Antibacterial Agent: Nanobubble Ozone Stored in Liposomes: The Antibacterial Activity of Nanobubble Ozone in Liposomes and Their Thymol Solutions
Author: P.E. Alkan, M.E. Gunes, C.Ozakin & A.U. Sabanci
ABSTRACT
Ozone is a highly effective oxidant. In its gaseous form, ozone is not a stable compound; however, stability and effectiveness are sustained for a long period, when stored in vegetable oils. In this study, solutions are prepared containing a combination of ozone nanobubbles, vegetable oils and emulsifiers. Throughout the study, the antibacterial effectiveness and stability of ozone nanobubble carried in liposomes and their solutions with thymol are tested on strains of Staphylococcus aureus (ATCC 25923) and Escherichia coli (ATCC 25922). Findings of the study suggest that the stability range of the ozone nanobubbles carried in liposomes extend to a 1-year period. In addition, the antibacterial effectiveness of this structure, both with and without thymol, is tested to be substantially high on the sample bacteria. These ozone structures/solutions can be stored at +4 degrees for an extended period of time compared to ozone’s possible stability range as a sole trioxygen molecule. Results of the study highlight the possibility of extending the storage/stability range even further.
https://www.tandfonline.com/doi/full/10.1080/01919512.2021.1904205
Impacts of oxygen and ozone nanobubbles on bacteriophage in aquaculture system
Authors: Le Thanh Dien, Nguyen Vu Linh, Thao Thu Mai, Saengchan Senapin, Sophie St-Hilaire, Channarong Ha Thanh Dong
ABSTRACT
Injection of ozone nanobubbles into water reduces bacterial load, improves dissolved oxygen, and modulates the fish innate immune system. Little is known about the effect that nanobubble treatment has on the concentration of viruses in water. This study, investigated the disinfection impact of oxygen and ozone nanobubbles (NB-O2 and NB-O3) on an Aeromonas hydrophila-specific phage, pAh6.2TG, a virus lab model. After 5-, 10- and 15-min treatments with NB-O2, the concentration of phage remained the same, while the same treatment with NB-O3 eradicated 99.99 to 100% of the phage in the water. Since this phage has been shown to control bacterial infections in fish, we further investigated whether NB-O2 improved the adherence of the phage to the body surface of the fish (i.e. skin mucus, and gills) and phage penetration into fish internal organs, specifically the liver. Nile tilapia, Oreochromis niloticus were used as experimental fish in this study. The results indicated that the number of phages adhered to the skin mucus and gills in NB-O2 treatment group was 1.07 to 15.0 times higher than in the untreated control group without gas nanobubbles. The phage uptake into fish liver after NB-O2 treatment increased 1.29 to 4.75 fold compared to untreated control. These findings suggested a plausible application of NB-O2 treatment for improving efficacy of phage therapy in aquaculture. On the other hand, NB-O3 application may be useful for disinfection of harmful viruses in culture water, but the application would need to be omitted during phage treatment. This study provides preliminary information on potential applications of nanobubble technology in aquaculture to reduce viral load in the water.
Introduction
Aquaculture is one of the fastest-growing food industries, and it plays a crucial role in global food security and nutrition, particularly in low- and middle-income countries (LMICs) (FAO, 2020; Hicks et al., 2019; Naylor et al., 2021; Webb et al., 2020). However, aquaculture sectors have faced increasing challenges with infectious diseases including antimicrobial resistance (AMR) in microorganisms (Stentiford et al., 2020; Stentiford et al., 2017). Thus, research efforts on non-chemical approaches to controlling pathogens, such as the use of nanobubbles (NBs) have increased in recent years to reduce the risk of AMR and address production losses caused by the emergence of pathogenic AMR bacterial strains (Hoelzer et al., 2018; Reverter et al., 2020; Watts et al., 2017).
Nanobubbles are bubbles less than 100 nm in diameter. They can be created with different gases, and have neutral buoyancy, which enables them to have a lengthy residence time in water (Agarwal et al., 2011; Tsuge, 2014). In aquaculture, oxygen nanobubbles (NB-O2) are commonly used for improving dissolved oxygen (DO) and promoting the growth of aquatic animals (Mahasri et al., 2018; Mauladani et al., 2020; Rahmawati et al., 2020). Recent studies have indicated that ozone nanobubbles (NB-O3) have potential to reduce pathogenic bacteria, improve DO in water, and modulate the immune systems of fish against bacterial infection (Dien et al., 2021b; Imaizumi et al., 2018; Jhunkeaw et al., 2021; Linh et al., 2021; Nghia et al., 2021). Hitherto, the effect of NB-O2 and/or NB-O3 on aquatic viruses remains uninvestigated.
Lytic bacteriophages (also known as phages) are viruses that infect and kill bacteria (Kutateladze and Adamia, 2010). Due to the high similarity to animal virus properties, phages have been considered as models for studies related to the survival of viruses under different environmental conditions and to evaluate the efficacy of disinfection methods (Grabow, 2001; Pinon and Vialette, 2018). On the other hand, phage therapy could also be used as a natural strategy to control bacteria to replace or supplement chemotherapy in aquaculture (Angulo et al., 2019; Culot et al., 2019; Rao and Lalitha, 2015; Richards, 2014). Several studies have revealed that the increase of phage binding to the mucosal layer of the host improved protection against bacterial infections (Almeida et al., 2019; Barr et al., 2013; Barr et al., 2015; Dabrowska et al., 2005). Given the properties of nanobubbles, we hypothesized that depending on the gas used this technology could either destroy phages or enhance their uptake into fish, which might improve their therapeutic function against bacterial diseases.
The effects of NB-O2 and NB-O3 treatments on phage concentration in water were explored in this study. Subsequently, we investigated whether NB-O2 treatment could improve adherence of phages to fish body surfaces and their uptake into the fish.
Discussion
Disinfection of NB-O3 against pathogenic bacteria has previously been investigated in both marine and freshwater. Imaizumi et al. (2018) reported that most Vibrio parahaemolyticus were killed after 1 min incubation in NB-O3 seawater. Nghia et al. (2021) also demonstrated that treatment with NB-O3 for 6 min inactivated 100% of the V. parahaemolyticus in laboratory experiments. In addition, Jhunkeaw et al. (2021) demonstrated that 10 min of NB-O3 treatment in freshwater reduced 96.11 and 97.92%
https://www.sciencedirect.com/science/article/abs/pii/S0044848622000084?via%3Dihub
Ozone nanobubble modulates the innate defense system of Nile tilapia (Oreochromis niloticus) against Streptococcus agalactiae
Authors:
ABSTRACT
Ozone nanobubble (NB–O3) is a promising technology for improving dissolved oxygen and reducing bacterial concentration in aquaculture systems. Here, we investigated the effects of NB-O3 on the innate immunity of fish by monitoring the expression levels of nonspecific immune-related genes (IL-1β, IL-2β, TNF-α), heat-shock protein genes (HSP70, HSP90-α), and a bacteriolytic enzyme, C-type lysozyme, gene (LYZ) post-treatment with this technology. Following exposure to NB-O3, the different tissues of Nile tilapia (Oreochromis niloticus) were collected over time for quantitative real-time PCR (qPCR) analysis. The expression of all the genes evaluated in the gills, the head kidney, and the spleen of the NB-O3 treated group was significantly up-regulated compared to that in the untreated control group. The expression levels were the highest (approx. 2 to 4-fold) at 15 min and 3 h post-exposure and then decreased from 6 to 24 h. These findings suggested that NB-O3 could switch on the innate immunity genes of Nile tilapia. Thus, we hypothesized that the NB-O3-immune-activated fish would respond more effectively to subsequent bacterial infections, thereby improving survivability compared to that of untreated fish. To test this hypothesis, 3 h post NB-O3 exposed fish and unexposed fish were challenged with a lethal dose of Streptococcus agalactiae. Interestingly, the survival rate of the NB-O3 group was significantly higher than that of the non-treated controls, with a relative percent survival (RPS) of 60–70%. Together, these findings indicate, for the first time, that NB-O3 may trigger the nonspecific defense system of the fish, thereby improving fish survivability during subsequent bacterial infections. This research identified another potential benefit of NB-O3 in aquaculture for preventing infectious bacterial diseases.
Introduction
Water bubbles can be classified into three groups based on their sizes and characteristics. Macrobubbles have diameter varies from 100 μm to 2 mm, which rapidly reach the water surface and burst. Microbubbles (MBs) are smaller than macrobubbles, with a diameter size between 1 μm and 100 μm, which shrink in the water and dissolve afterward. By contrast, nanobubbles (NBs) or ultrafine bubbles are incredibly tiny gas bubbles with diameter < 1 μm, high internal pressure, negatively charged, and low buoyancy [1]. These distinctive physical properties allow NBs to be suspended for long periods in liquids. They exhibit high specific surface areas and high stagnant periods, which enhance the efficacy of mass transportation, physical uptake processes, and chemical reactions at the gas-liquid interface [1,2]. Therefore, NBs technology has been extensively explored in numerous fields, including engineering, medical, agriculture, food, and aquaculture [[3], [4], [5], [6], [7]].
Nanobubble technology is relatively new to the aquaculture industry. There are only a few studies describing the manner in which the unique properties and stability of NBs may be utilized to enhance growth performance of cultivated animals and reduce pathogen load in water [8]. Kurita et al. [9], showed that the survival rate of planktonic crustacean parasites in aquaculture tanks was decreased by approximately 63% following exposure to NB-O3 for 25 min compared to that of the control groups. In addition, 4 d after exposure to NB-O3, no negative impact on the survival rate of juvenile sea cucumbers (Apostichopus japonicus) and sea urchins (Strongylocentrotus intermedius) was observed, suggesting that NB-O3 treatment is relatively safe for these marine aquaculture species. NB-O3 is also effective at disinfecting Vibrio parahaemolyticus in brackish water, and preventing mortality caused by this pathogen in whiteleg shrimp (Penaeus vannamei) [10]. Our recent investigation on the acute impact of NB-O3 on Nile tilapia and its pathogens demonstrated that a 10-min NB-O3 treatment (834 ± 22 mV ORP) was effective at reducing the concentration of Streptococcus agalactiae and Aeromonas veronii in freshwater, and caused no acute mortality to Nile tilapia [21]. The use of NB-O3 in tilapia aquaculture is promising, however, effects of NB-O3 on the fish immune system remain uninvestigated.
Innate immunity, which constitutes front-line defense against infections, refers to a critical systemic response that inhibits pathogens and sustains homeostatic interactions [[11], [12], [13]]. Significant differences in the expression transcripts of nonspecific immune-related genes following a pathogenic infection are important indicators of the immune response in fish. Thus, studies of the nonspecific immune-related genes may lead to a better understanding of the immune mechanisms in finfish. In a continuous effort to understand how nanobubble technology affects fish health, we investigated the effects of NB-O3 on the innate immunity of fish by monitoring the expression of nonspecific immune-related genes: interleukin 1 beta (IL-1β); interleukin 2 beta (IL-2β); tumour necrosis factor alpha (TNF-α), which regulate pro-inflammatory cytokines [[14], [15], [16], [17], [18]], heat-shock protein genes (HSP70, HSP90-α), which are now supposed to play a larger-term function by modulating the immune system [19], and a bacteriolytic enzyme, C-type lysozyme gene (LYZ), which destroys the bacterial cell wall [20]. Subsequently, we determined whether NB-O3 treatment improves survival rate of Nile tilapia following infection with S. agalactiae.
Discussion
Current application of NB-O3 in aquaculture is still limited due to lack of knowledge of the advantages of this technology. Previous studies reported several benefits of NB-O3 in respect of reducing concentration of certain pathogenic bacteria and increasing dissolved oxygen (DO) in water while not negatively impacting the health of aquaculture species [21,24,25]. In a continuous effort to explore further benefits of this technology in aquaculture, this study discovered that a short exposure of
https://www.sciencedirect.com/science/article/abs/pii/S1050464821000565
An integrated approach using ozone nanobubble and cyclodextrin inclusion complexation to enhance the removal of micropollutants
Authors: Wei Fan, Wengang An, Dan Xiao, Tao Lyu, Jingyu Cui, Mingxin Huo
Abstract
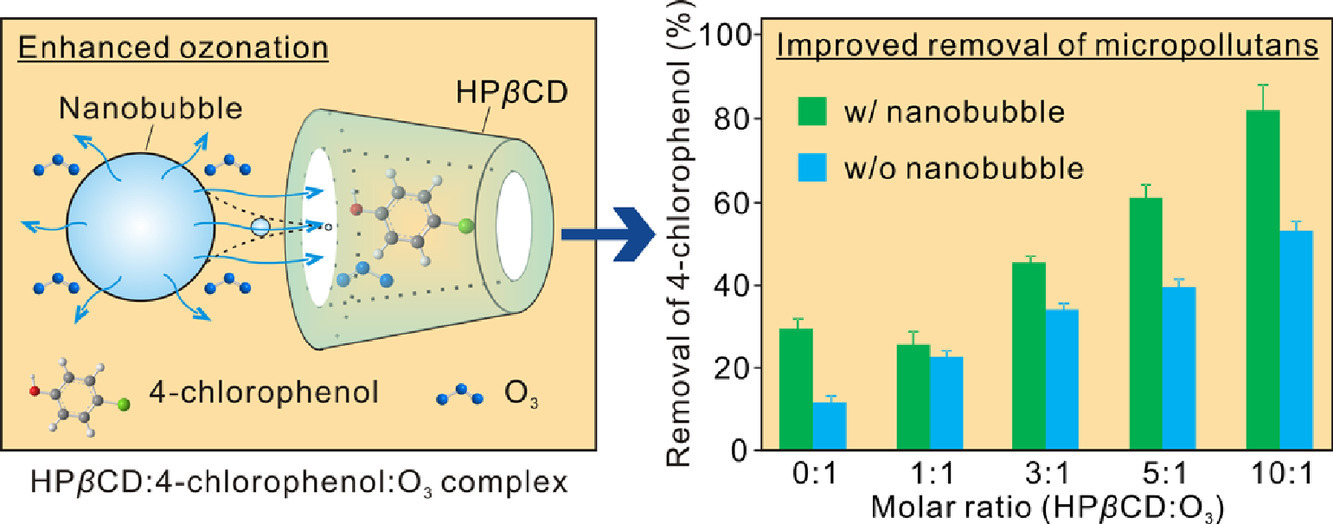
Ozone (O3) has been widely used for the elimination of recalcitrant micropollutants in aqueous environments, due to its strong oxidation ability. However, the utilization efficiency of O3 is constrained by its low solubility and short half-life during the treatment process. Herein, an integrated approach, using nanobubble technology and micro-environmental chemistry within cyclodextrin inclusion cavities, was studied in order to enhance the reactivity of ozonisation. Compared with traditional macrobubble aeration with O3 in water, nanobubble aeration achieved 1.7 times higher solubility of O3, and increased the mass transfer coefficient 4.7 times. Moreover, the addition of hydroxypropyl-β-cyclodextrin (HPβCD) further increased the stability of O3 through formation of an inclusion complex in its molecule-specific cavity. At a HPβCD:O3 molar ratio of 10:1, the lifespan of O3 reached 18 times longer than in a HPβCD-free O3 solution. Such approach accelerated the removal efficiency of the model micropollutant, 4-chlorophenol by 6.9 times, compared with conventional macrobubble ozonation. Examination of the HPβCD inclusion complex by UV–visible spectroscopy and Nuclear Magnetic Resonance analyses revealed that both O3 and 4-chlorophenol entered the HPβCD cavity, and Benesi-Hildebrand plots indicated a 1:1 stoichiometry of the host and guest compounds. Additionally, molecular docking simulations were conducted in order to confirm the formation of a ternary complex of HPβCD:4-chlorophenol:O3 and to determine the optimal inclusion mode. With these results, our study highlights the viability of the proposed integrated approach to enhance the ozonation of organic micropollutants.
Graphic Abstract
Introduction
Organic micropollutants, such as pesticides (Lv et al., 2016), pharmaceutical and personal care products (Zhang et al., 2018), have been extensively detected in various aquatic systems, and pose a serious risk to the environment and to human health. A majority of such emerging contaminants have been reported to only achieve limited treatment efficiencies by conventional wastewater treatment plants, and thus development of alternative approaches toward more effective removal technologies is urgently required (Falås et al., 2016; Lyu et al., 2018). Due to a superior oxidation potential (2.07 V), the widely used ozone (O3) treatment technology exhibits high capability for the oxidation of pharmaceuticals in drinking water (Prasse et al., 2012), remediation of antibiotics in wastewaters (Alexander et al., 2016), and removal of recalcitrant organic pollutants in groundwater (Hu and Xia, 2018). Molecular O3 not only oxidises such pollutants directly, but also acts as a precursor to generate the hydroxyl radical (•OH) that is even more reactive as a nonselective oxidant (Kim et al., 2020). Nevertheless, efficient ozonisation is still restricted by low water solubility (e.g. < 10 mg l-1 in typical groundwater) and short half-life (typically < 1 h) (Khan and Carroll, 2020), and remains a significant technological challenge.
Many efforts have been made to enhance the ozonation process, including the addition of peroxides and thiosulfate (Yang et al., 2020), coupling ozonation with photocatalytic/electrolytic processes (Mehrjouei et al., 2015), and catalysis by metal ions or metal oxides (Nawrocki and Kasprzyk-Hordern et al., 2010). These approaches mainly focus on promoting the generation of •OH from O3, thus resulting in better oxidation of refractory organic contaminants (Yang et al., 2020). However, such optimisations still do not increase the solubilisation or prolong the reactivity of aqueous O3.
Nanobubbles are defined as bubbles with a diameter of less than 1000 nm and possess special characteristics compared with normal macrobubbles, such as low buoyancy and long lifetimes (Zimmerman et al., 2011; Wang et al., 2020). In addition, the smaller bubbles have a greater surface area per unit volume and could therefore increase the gas transfer rate into the surrounding water, significantly improving O3 dissolution (Parmar and Majumder, 2015). Previous studies have demonstrated that the mixture of micro- and nanobubbles could increase the mass transfer coefficient (KLa) of O3 by 2 times higher than that of macrobubbles (Gao et al., 2019). Moreover, it has been reported that nanobubbles can exist in water for days due to their low buoyancy (Ghaani et al., 2020). These unique characteristics of O3 nanobubbles have been used to enhance the in-situ remediation of trichloroethylene-contaminated groundwater (Hu and Xia, 2018). Although the improvement of O3 dissolubility and mass transfer rate through nanobubble technology could facilitate the ozonation process, the short half-life caused by the undesirable consumption of the oxidant, i.e. O3 and •OH, by non-target compounds and radical scavengers (Gardoni et al., 2012; Yuan et al., 2020) still limit the utilisation efficiency of O3.
Currently, some chemical stabilisers have been developed to address this issue. amongst them, addition of gentle organic acids such as acetic, propionic and short-chain fatty acids have been proven to increase the half-life of O3 during ozone-based treatments (Alcantara-Garduno et al., 2008; Britton et al., 2020). Fan et al. (2020) reported that complete attenuation of O3 could take 2.5 h in 5% acetic acid solution (pH = 2.56), which is 100 min longer than in pure water. Nevertheless, these acidic effluents would need further treatment before discharge, which would incur considerable hidden costs for the application. Another promising method was through use of cyclodextrins (CDs), which contain doughnut-shaped molecular structures with a hydrophilic outer shell and a hydrophobic inner cavity (Cai et al., 2015; Dettmer et al., 2017; Khan et al., 2018). This low polarity cavity can encapsulate dissolved size-matched guest compounds via coordination through various weak interactions (e.g. hydrophobic interactions, hydrogen bonds, and steric effects) and features molecular recognition capabilities (Fernández et al., 2019; Liu et al., 2020). Such inclusion complexes could increase the stabilisation of the enclosed molecules and prolong their lifespan, which would have the effect of also increasing the apparent solubility of low polarity organic compounds and thus their availability for removal (viz. sorption or degradation) (Cheirsilp and Rakmai, 2016; Zhou et al., 2018, 2020). According to this concept, Dettmer et al. (2017) initially investigated the hydroxypropyl-β-cyclodextrin (HPβCD):O3 clathrate complex as an ozone stabilizer. The addition of HPβCD has been further proven to significantly enhance the ozonation of 1,4-Dioxane, Trichloroethylene and 1,1,1-Trichloroethane (Khan et al., 2019). Nevertheless, the molecular structures of the complexes and the dynamics of their formation need further study in order to reveal the underpinning mechanisms.
The purpose of this study was to investigate the combined approach of using O3 nanobubbles and HPβCD to overcome the dual problems hitherto inherent in the ozonation process for remediation of organic pollutants. HPβCD, as a cost-effective and eco-friendly model cyclodextrin (Gloud and Scott, 2005), was selected to evaluate the proposed approach for micropollutants remediation. The main hypothesis is that the O3 nanobubbles could increase the solubility and transfer efficiency of O3, then the dissolved ozone could enter into the cyclodextrin cavity which would inhibit their otherwise rapid decay. Therefore, in this study, the improvement of O3 solubility and mass transfer rate, through application of nanobubble technology, was first examined. Furthermore, different HPβCD:O3 molar ratios were utilised in order to investigate the lifespan of the complexed O3, and the dynamics of decay of the generated reactive oxygen species (ROSs), i. e. •OH and H2O2. The removal performance and degradation kinetics of 4-chlorophenol, a model micropollutant, were monitored in order to further evaluate the proposed integrated ozonation technique. In order to understand the mechanisms, UV-visible spectroscopy (UV–vis) and nuclear magnetic resonance (NMR) analysis were used to characterise the HPβCD inclusion complex and molecular docking simulations were conducted to reveal probable modes of inclusion formation.
Discussion
This study developed a promising approach by coupling nanobubble technology and the addition of HPβCD in order to enhance the ozonation of an organic micropollutant in artificial contaminated water. Nanobubbles were found to promote the solubilisation and mass transfer of O3, and also benefitted from the formation of inclusion complexes in the cavities of HPβCD, compared with conventional macrobubbles. The presence of HPβCD contributed to the stabilisation of O3 by inclusion complexation,
https://www.sciencedirect.com/science/article/abs/pii/S0043135421002372